Abstract
Background: Responses to FLT3-inhibitors are usually transient due to emergence of resistance through the acquisition of kinase domain point mutations and other non-mutational mechanisms. SEL is a potent first-in-class Selective Inhibitor of Nuclear Export/SINE™ that exerted marked cell killing of human and murine FLT3 -mutant AML cells, including those with ITD, D835Y, ITD+Y842C or ITD+F691L mutations by modulating the cdk inhibitor p27 and anti-apoptotic Mcl-1 (Zhang W et al, Blood 2015 126). The combination of SEL+sorafenib had synergistic pro-apoptotic effects in FLT3-mutated AML cells by suppressing phosphorylation levels of FLT3 and its downstream signaling mediators ERK/AKT, and by inducing myeloid differentiation in ITD and D835 mutated cell lines.
Methods: We designed a phase I/II trial of SEL with sorafenib for FLT3-ITD and/or -D835 R/R AML pts. In phase I, the primary objectives were to determine the maximum tolerated dose (MTD), the recommended phase 2 dose (RP2D), and dose-limiting toxicity (DLT) of the combination. In phase II, primary objectives included the rate of complete remission (CR) + CR with incomplete count recovery (CRi) + partial response + >50% blast reduction within 3 months of therapy initiation. Secondary objectives were safety and overall survival (OS). SEL was given orally twice a week for 3-weeks on and 1-week off in 28-day cycles and sorafenib was given continuously at a dose of 400mg twice daily from cycle 1 day 1 of SEL. In phase I, SEL dose was de-escalated in "3+3" fashion starting at dose level 0 and going down if DLTs were exceeded (dose levels 0, -1, -2 = 80, 60, 40 mg, respectively). Once MTD was established, the phase II enrolled pts in 2 cohorts: prior FLT3-inhibitor failure (cohort 1) and FLT3-inhibitor naïve (cohort 2). PB or BM samples were collected at pre-dose (C1D1), 24 h (C1D2) and day 28 (C1D28) of cycle 1, and apoptosis induction was determined by measuring annexin V positivity with FACS.
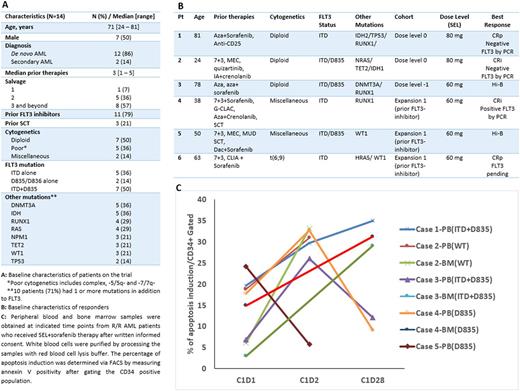
Results: Fourteen pts with a median age of 71 years (range, 24-81) were enrolled. All pts had baseline next generation sequencing for AML specific mutations (Figure 1A). The median number of prior therapies was 3 (range, 1-5) as follows: salvage (S) 1: n=1, S2: n=5, S3+: n=8. 11 (79%) pts had prior FLT3-inhibitors: 1 prior FLT3-inhibitor (n=9); 2 prior FLT3-inhibitors: n= (2). Three pts had prior SCT. Four pts were treated at dose level 0 (SEL 80mg) with DLTs in 2 (Grade (G) 3 sepsis, n=1; G3 mucositis, syncope, adrenal insufficiency, n=1). Three pts were subsequently treated at dose level -1 (SEL 60mg) with no DLTs and this was established as the MTD/RP2D. Seven pts were treated in expansion: 6 had prior FLT3-inhibitor therapy (cohort 1) and 1 had no prior FLT3-inhibitor (cohort 2).
The median duration of treatment for all 14 pts was 28 days (range, 14-122) and therapy is ongoing in 1 pt. All pts are evaluable for response. CRp/CRi and >50% blast reduction as best responses were seen in 4/14 (29%) and 2/14 (14%) pts, respectively. All 6 responders had a prior FLT3 inhibitor yielding a response rate of 55% (6/11) pts with prior FLT3 inhibitor therapy. 3 pts with CRp/CRi (2 with FLT3-ITD and 1 with ITD+D835) achieved negative RT-PCR for FLT3. (Figure 1B). One pt in CRi was bridged to SCT and remains alive at 1 year. The most common G 3/4 adverse events irrespective of causality were bleeding (gastrointestinal, n=4; intracranial, n=1), febrile neutropenia (n=4), pneumonia (n=3) and syncope (n=2). The reasons for discontinuing study therapy were disease progression (n=9; 64%), toxicity (n=3; 21%) and SCT (n=1; 7%). The median OS for pts on study was 3.5 months (range, 0.9-15). Translational analysis showed that SEL+sorafenib induced apoptosis in 4 of 5 tested patients, especially in the BM samples (Figure 1C)
Conclusions: The combination of SEL and sorafenib is safe with clinical activity and apoptosis induction in R/R FLT3+ AML. The benefit was especially encouraging in pts exposed to prior FLT3 inhibitors, with a response rate of 55% (6/11; including 2 CRi and 2 CRp). The RP2D is 60 mg twice weekly of SEL and the study is accruing (NCT01607892).
Daver: Kiromic: Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Otsuka America Pharmaceutical, Inc.: Consultancy; Incyte Corporation: Honoraria, Research Funding; Pfizer Inc.: Consultancy, Research Funding; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Jazz: Consultancy; Immunogen: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. DiNardo: Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Cortes: BMS: Consultancy, Research Funding; Sun Pharma: Research Funding; ARIAD: Consultancy, Research Funding; Teva: Research Funding; Pfizer: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding. Kantarjian: Novartis: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal